Abstract
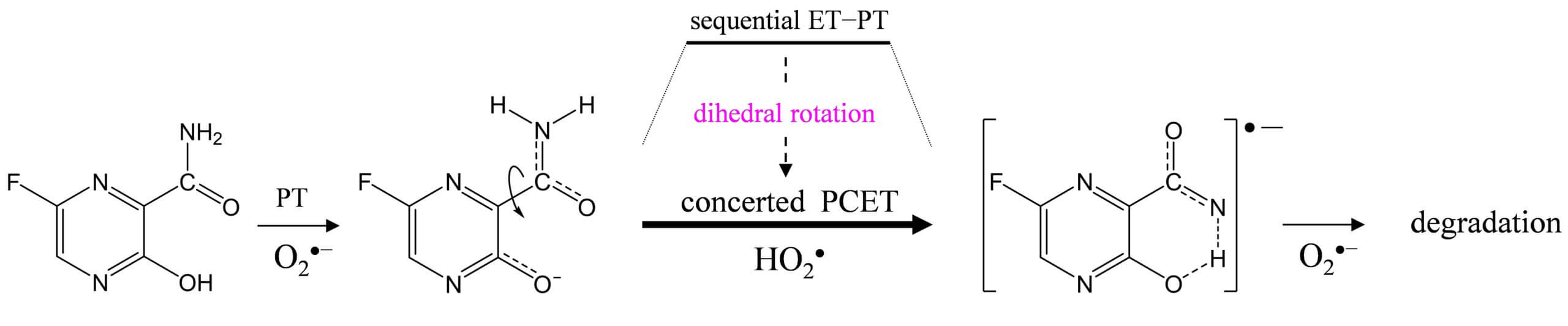
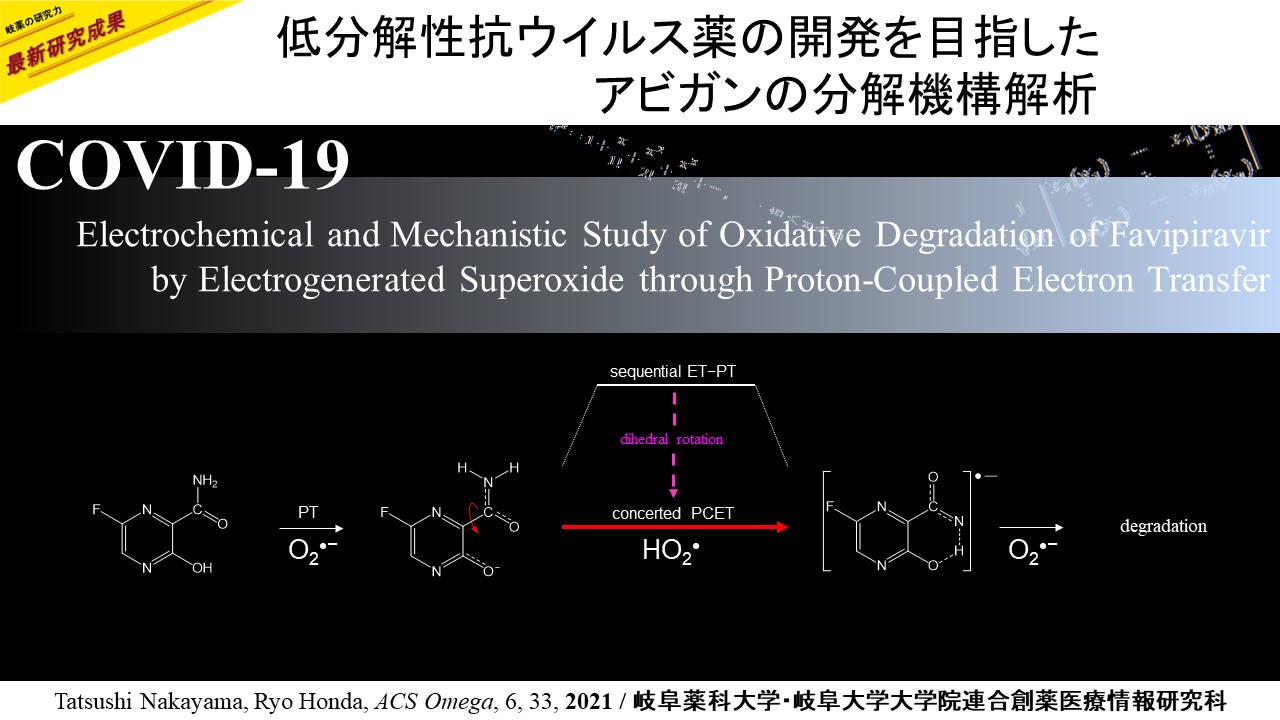
Electrochemical analyses aided by density functional theory calculations were used to investigate the oxidative degradation of pyrazine antiviral drugs, 3-hydroxypyrazine-2-carboxamide (T-1105) and 6-fluoro-3-hydroxypyrazine-2-carboxamide (favipiravir, T-705), by the electrogenerated superoxide radical anion (O2•–). T-1105 and T-705 are antiviral RNA nucleobase analogues that selectively inhibit the RNA-dependent RNA polymerase. They are expected as a drug candidate against various viral infections, including COVID-19. The pyrazine moiety was decomposed by O2•– through proton-coupled electron transfer (PCET). Our results show that its active form, pyrazine-ribofuranosyl-5′-triphosphate, is easily oxidized under inflamed organs by overproduced O2•– through the PCET mechanism in the immune system. This mechanistic study implies that the oxidative degradation of pyrazine derivatives will be prevented by controlling the PCET through simple modification of the pyrazine structure.
Research results
-
Pyrazine antiviral drugs are easily decomposed by O2•– through the PCET involving two PTs and one ET.
-
The concerted PCET after the initial PT occurs in one kinetic process via a TS without forming a high-energy intermediate.
-
The net reaction involves rotation of the dihedral angle around the 2-carboxamide group along the PCET.
Future work
Paper
- Electrochemical and Mechanistic Study of Oxidative Degradation of Favipiravir by Electrogenerated Superoxide through Proton-Coupled Electron Transfer
- ACS Omega 2021, 6, 33, 21730–21740
- Tatsushi Nakayama, Ryo Honda(中山辰史、本田諒)
DOI: doi.org/10.1021/acsomega.1c03230 - Preprint 2021/06
10.33774/chemrxiv-2021-gfrq5
- A Mechanistic Insight into Oxidative Degradation of Favipiravir by Electrogenerated Superoxide through Proton-Coupled Electron Transfer
-
Research Trends and Challenges in Physical Science Vol. 7, 4 March 2022 , Page 9-25
- tatsushi nakayama, Ryo Honda
-


