
Electrochemistry (E-chem)

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electrical potential as an outcome of a particular chemical change, or vice versa. Our laboratory mainly deals with electrochemical measurements in solution and analyzes the information obtained from the current-voltage curves measured by a three-electrode system (working electrode, counter electrode, reference electrode). From the obtained current-voltage curve, we analyze the state of oxidation and reduction of substances from the viewpoint of thermodynamics and reaction kinetics. In electrochemical measurement, it is also possible to analyze the chemical reaction (electro-chemical) associated with electron transfer, and it is possible to analyze the substance parameters in the solution including the analysis of mass transfer.
⇒ Electrochemistry of dioxygen
E-chem in aprotic solvent
Reaction analysis in a proton-free solution is possible in a non-aqueous (aprotic) solution. For example, when analyzing electron transfer in water, electron transfer and accompanying proton transfer (hydrogen bond with water molecule) have a non-negligible effect, so we are not observing the true electron transfer to be analyzed. We are only observing reactions via interactions with water molecules (solvation). Therefore, in order to analyze the reaction correctly, it is essential to analyze the reaction in an aproton-based (non-aqueous) solvent that completely eliminates the effects of water and protons.
⇒ electrochemistry in aprotic solution
CV simulation
By simulation analysis of electrochemical data such as CV, electron transfer, chemical reaction, mass transfer (diffusion, etc.) in solution are parameterized. The PC compatible computer program is used for a simulation-based approach of the analysis of cyclic voltammograms.
In-situ electrolytic ESR/UV-Vis spectrometry
In situ Electron Spin Resonance measurements(ESR) / ultraviolet-visible spectrometry(UV-Vis)
Computational chemistry/biochemistry
ab initio calculation
- molecular orbital calculation
- density functional calculation
- molecular dynamics
- quantum chemical calculation
semi-empirical calculation
ONIOM(our own N-layered integrated molecular orbital and molecular mechanics)法
docking simulation
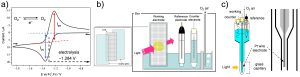
Electrochemistry of dioxygen
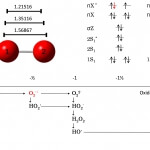
Cyclicvoltammetry of O2/O2•− in aprotic solventFig.1. Cyclic voltammograms f...
続きを読む実験設備・装置
電気化学関連設備・装置スタンダード電気化学分析セルVB1 / EC-Frontier20 mL / 50 mL 密閉型セルスタンド付で目視しながら測定できます...
続きを読むComputational Chemistry
当研究室では、化学実験と並んで、取得データの解析・理解に計算科学的手法(in silico)を用いています。数値データについて、数学的モデルとその定量的評価法を構築し、あるいは計...
続きを読む

