For electrochemical measurements in non-aqueous (aprotic) solutions, a two-electrode or three-electrode potentiostat connected in a solution of a polar organic solvent such as acetonitrile (CH3CN), N,N-dimethylformamide (DMF), or dimethyl sulfoxide (DMSO) with an electrolyte is used.
The obtained current-voltage curve shows not only electron transfer but also chemical reactions in solution, which is useful for reaction analysis.
⇒ Reference) EC-frontier.com (https://ec-frontier.co.jp/)
Three electrode system

CV was recorded via a standard three-electrode system comprising a 1.0-mm diameter glassy carbon (GC) working electrode, a coiled platinum (99.99%) counter electrode, and an Ag/AgNO3 reference electrode (containing CH3CN solution of 0.1 mol dm−3 tetrabutylammonium perchlorate and 0.01 mol dm−3 AgNO3; BAS RE-5) at 298 K.
Prior to all experiments, the working electrode was polished on a polishing wheel with alumina paste. The electrode was then rinsed with deionized water and acetone. After air-drying, the electrode was ready to use. The reference electrode was calibrated with reference to the ferrocenim ion/ferrocene couple (Fc+/Fc), and all potentials reported here are referenced to the potential of this couple.
Solvents and electrolyts
Spectrum-grade anhydrous aprotic solvents (acetonitrile, DMF, DMSO, and so on) used in the electrochemical were used. Tetrapropylammonium perchlorate (TPAP, >98.0%, Tokyo Chemical Industry Co., Ltd.) and tetrabutylammonium perchlorate (TBAP, >98.0%, Tokyo Chemical Industry Co., Ltd.) were used as the supporting electrolyte for the measurements and was recrystallized several times, dried overnight at 313.0 K under reduced pressure, and dried again for 2 h or more immediately before use.
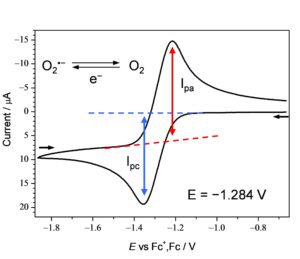
Cyclicvoltammetry: CV
CV measurement, which is the most popular method among various electrochemical methods, can also analyze the homogeneous chemical reaction of redox substances after electron transfer in an aprotic solvent, such as EC-mechanism (electron-chemical transfer reaction). The electron transfer of the product is called the ECE reaction (Electro-Chemical-Electro), and it is an excellent tool for accurately analyzing the chemical reaction of high-energy-intermediates that follows the oxidation-reduction electron transfer.
Digital simulation of cyclicvoltammograms
Digital simulations of CV are performed using computer simulation software.