PCET反応を介したピロガロールによるスーパオキサイド消去に関する研究
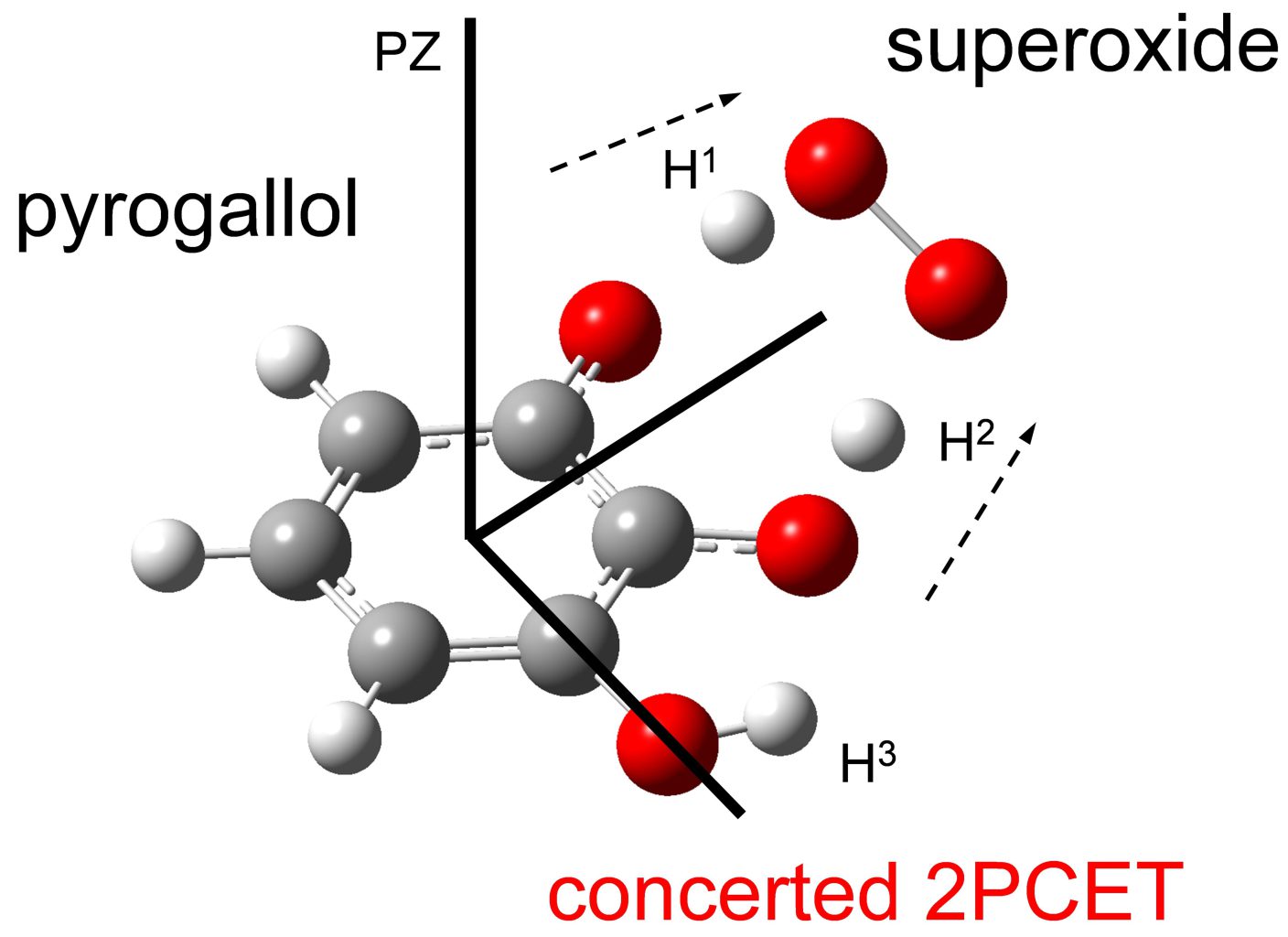
Scavenging of electrogenerated superoxide radical anion (O2•−) by pyrogallol (PyH3) was investigated on the basis of cyclic voltammetry and in situ electrolytic electron spin resonance spectrum in N,N-dimethylformamide with the aid of density functional theory (DFT) calculations. Quasi-reversible dioxygen/O2•− redox couple was modified by the presence of PyH3, suggesting that O2•− was scavenged by PyH3 through proton-coupled electron transfer (PCET) involving two proton transfer and one electron transfer. DFT calculation suggested that the pre-reactive formation of a hydrogen-bond (HB) complex and the subsequent concerted two-proton-coupled electron transfer characterized by catechol moiety in PyH3 is plausible mechanism that embodies the superior kinetics of the O2•− scavenging by PyH3 as shown in the electrochemical results. Furthermore, it was clarified that the three hydroxyl groups of PyH3 promote the formation of HB complex, in comparative analyses using related compounds, resulting in the promotion of the O2•− scavenging.
Keywords: proton-coupled electron transfer; superoxide radical anion; antioxidants; cyclic voltammetry; electron spin resonance spectrum; pyrogallol
Electrochemical and Mechanistic Study of Superoxide Scavenging by Pyrogallol in N,N-Dimethylformamide through Proton-Coupled Electron Transfer
Tatsushi Nakayama, Ryo Honda, Kazuo Kuwata, Shigeyuki Usui and Bunji Uno
Electrochem 2022, 3(1), 115-128
DOI: doi.org/10.3390/electrochem3010008