論文がアクセプトされました(J. Agri. Food, Chem., IF=5.9)
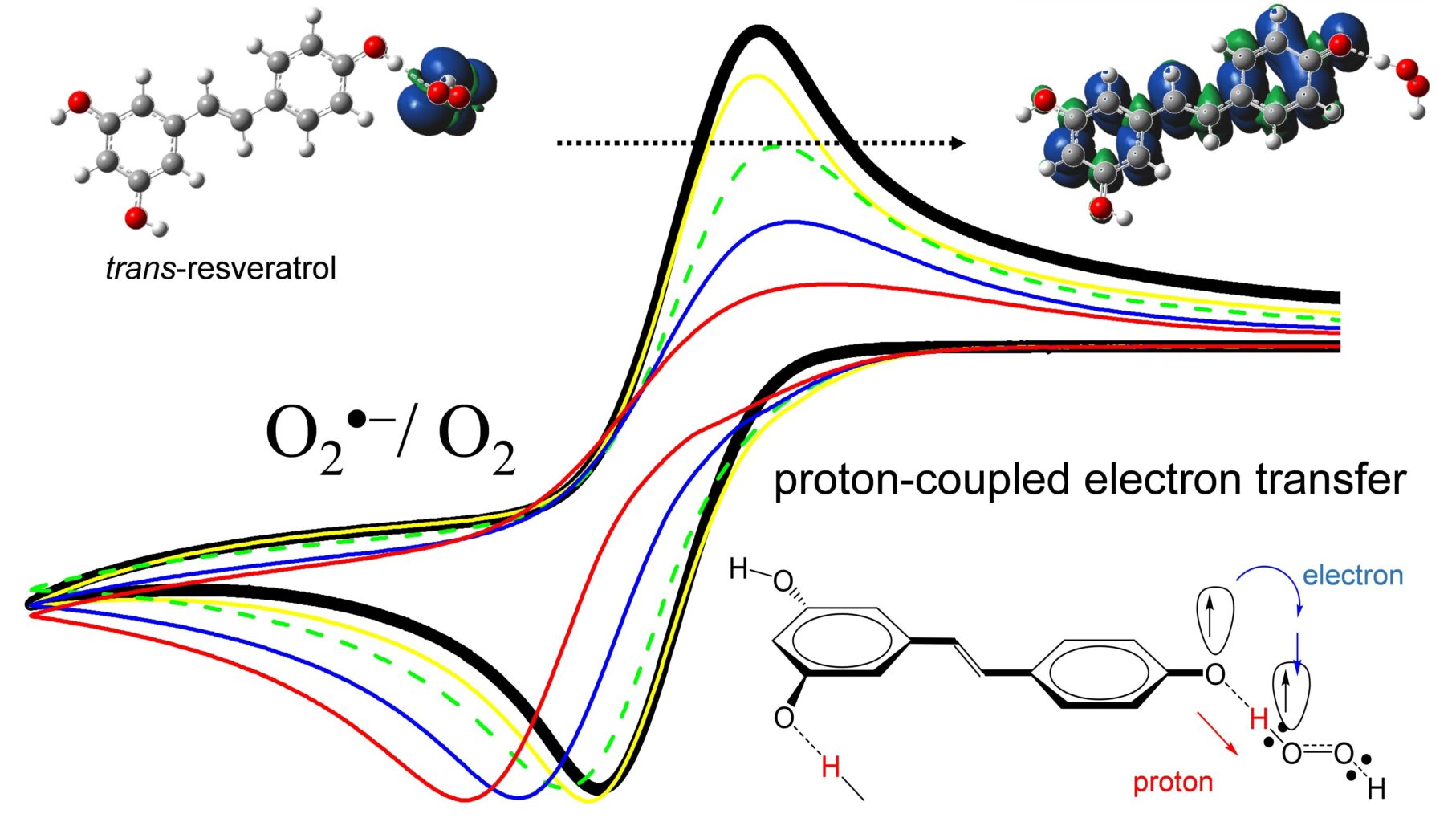
論文名「Reactivity of trans-Resveratrol toward Electrogenerated Superoxide in N,N-Dimethylformamide」がJournal of Agricultural and Food Chemistry誌(American Chemical Society)に掲載されました。
The reactivity of 5-[(E)-2-(4-hydroxyphenyl)ethen-1-yl]benzene-1,3-diol (trans-resveratrol) and related compounds toward electrogenerated superoxide radical anion (O2•–) were investigated using electrochemistry, in situ electrolytic electron spin resonance, and in situ electrolytic ultraviolet–visible spectral measurements, in N,N-dimethylformamide (DMF) with the aid of density functional theory (DFT) calculations. The quasi-reversible cyclic voltammogram of dioxygen/O2•– was modified by the presence of trans-resveratrol, suggesting that the electrogenerated O2•– was scavenged by trans-resveratrol through proton-coupled electron transfer (PCET) via three phenolic hydroxy groups (OH) on the stilbene moiety. The reactivity of trans-resveratrol toward O2•– characterized by the OHs was experimentally confirmed in comparative analyses using some related compounds, pinosylvin, pterostilbene, p-coumaric acid, and so on, in DMF. The electrochemical and DFT results suggested that a concerted PCET mechanism via 4′OH of trans-resveratrol proceeds, where the coplanarity of the two phenolic rings in the stilbene moiety linked by an ethylene bridge is essential for a successful O2•– scavenging.
Tatsushi Nakayama, and Bunji Uno
Journal of Agricultural and Food Chemistry 2023, impress
DOI: doi.org/10.1021/acs.jafc.2c08105




